Darwin’s Eye Problem Was Worse Than He Thought
Multiplying the Implausible
Darwin admitted that the eye was a ‘grave difficulty’ for his theory. More than a century later, the problem has not been solved — it has become worse than he imagined. The diversity of eyes across animals is so great that specialists now concede they must have arisen independently dozens of times. What Darwin saw as one difficulty has multiplied into many.
The human eye is a tightly integrated opto-neural control system. Its optical train begins before the cornea, with a three-layer tear film that sets the first refractive surface and keeps the corneal epithelium smooth. The lipid layer from Meibomian glands limits evaporation, the aqueous layer supplies oxygen and antimicrobials like lysozyme and lactoferrin, and the mucin layer wets the hydrophobic corneal surface. Disturb any layer and image quality collapses through scatter, drying, or epithelial damage. Behind that sits the cornea, a transparent composite whose collagen lamellae are precisely ordered; endothelial ion pumps pull fluid out of the stroma to prevent swell and haze. The cornea provides most of the eye’s refractive power.
The iris sets the aperture through the pupil, controlling light and depth of field, while the ciliary body adjusts the lens via zonular fibers to keep images in focus across distances. The lens itself is a graded-index element made of long-lived crystallin proteins that are packed to eliminate organelles and scatter, with α-crystallins acting as molecular chaperones to keep the lens clear for decades. Even slight failure in endothelial pumping, tear dynamics, or lens protein homeostasis yields blur or blindness. Each element depends on the others to deliver a stable wavefront to the retina.
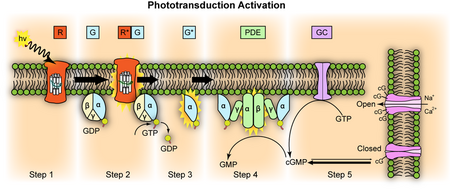
Phototransduction converts that wavefront into quantized signals with extreme sensitivity and speed.
Phototransduction is the process that takes place inside rods and cones, the photoreceptor cells of the retina. In their outer segments are stacks of disc-like membranes densely packed with visual pigment molecules — opsins bound to retinal. When even a single photon strikes one of these pigments, it flips the retinal and activates rhodopsin. This sets off a chemical cascade: rhodopsin triggers the G-protein transducin, which activates phosphodiesterase, which lowers cGMP, closing cGMP-gated ion channels and hyperpolarizing the cell. The effect is astonishing: one photon produces a measurable electrical signal. The cascade is designed for massive gain while keeping noise low, and it resets in milliseconds through calcium feedback, arrestin, rhodopsin kinase, guanylate cyclase, and RGS proteins. Cones sustain rapid color vision in bright conditions; rods extend sensitivity down to the single-photon level in dim light. But without the full set of enzymes and channels, the system fails. Remove PDE6 and rods saturate, remove the ion channels and there is no dark current to modulate, remove arrestin and recovery stalls. These are not interchangeable pieces; they are matched components of one irreducible circuit.
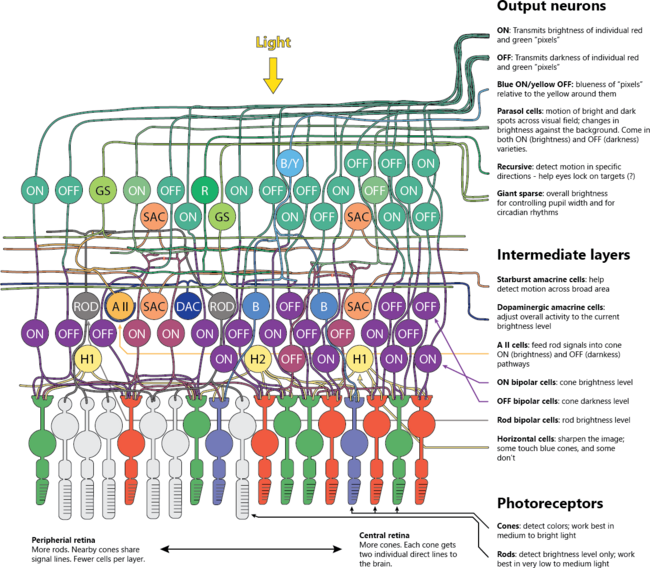
The retina performs layered preprocessing before a single axon leaves the eye. Horizontal cells shape photoreceptor output to create center-surround receptive fields that extract contrast. Bipolar cells split signals into ON and OFF pathways so increases and decreases in light are encoded separately.
Neuron circuits in the retina: ON and OFF pathways.
Amacrine circuits add temporal filtering, motion sensitivity, and direction selectivity. Ganglion cells compress the scene into parallel channels: high-acuity parvocellular, fast luminance magnocellular, color-opponent streams, and intrinsically photosensitive ganglion cells that carry irradiance for circadian timing and pupil control. This front-end compression lowers bandwidth while preserving behaviorally critical features. Break the ON pathway and night vision and contrast detection degrade; damage to specific amacrine circuits erases motion cues; disrupt melanopsin cells and circadian and pupil reflexes drift. Function depends on the full network.
The retinal pigment epithelium is the eye’s indispensable service bay. It forms the outer blood-retina barrier, regenerates 11-cis retinal through the retinoid cycle, phagocytoses spent outer-segment discs daily, recycles lipids, and manages ion and water flux for photoreceptor health. Choroidal circulation behind it delivers extraordinary oxygen, since photoreceptors are among the most metabolically demanding cells in the body. Starve the RPE of its enzymes, for example RPE65, and rods and cones fail even if optics and neurons are perfect. Compromise the barrier and toxic by-products accumulate, light scatter rises, and degeneration follows. The photoreceptors and RPE are a coupled machine; neither operates alone.
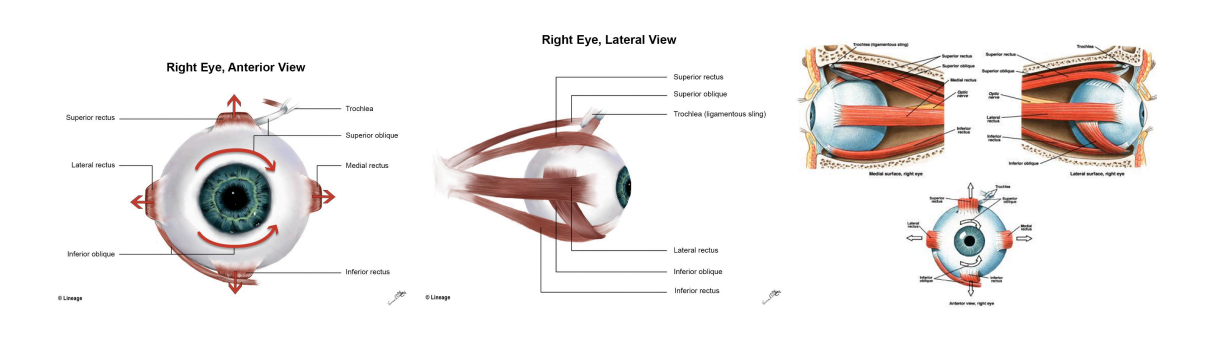
Precision sensing is worthless without stabilization and control. Six extraocular muscles, cranial nerves III, IV, and VI, vestibular inputs, cerebellar calibration, and cortical control produce saccades, smooth pursuit, vergence, and the vestibulo-ocular reflex that holds the scene steady when the head moves.
Microsaccades refresh the image on the fovea to prevent neural adaptation from erasing detail. The Edinger-Westphal nucleus drives the pupil light reflex; the ciliary muscle adjusts accommodation in a feedback loop that marries retinal blur signals to lens power. Damage any leg of this control stack and vision smears or oscillates even if optics and photoreceptors are pristine. Stabilization, gain control, and focus are not add-ons; they are core to seeing.
Figure from Baden & Nilsson, Is our retina really upside down?, Current Biology 32, R459–R461 (2022). The diagram illustrates a hypothetical evolutionary sequence for spatial vision: primitive photoreceptors without focus (A), crude image formation with a simple lens (B), and high-resolution focused vision with an enlarged eye and space for dense photoreceptors (C). The authors present this as evidence of how “evolution” solved optical problems, effectively treating evolution as a great designer. Yet for decades the inverted retina was invoked as a talking point against design. This same system is now defended as functional, but only by crediting blind chance with foresight.
For decades, prominent evolutionists (Dawkins, Coyne, Shubin, and many others) have used the “inverted retina” as one of their sharpest talking points. They presented it as a clumsy, backwards design that no intelligent engineer would ever choose, and therefore as proof that the eye was a product of blind evolutionary trial and error. That rhetoric promulgated into popular books, classrooms, and even memes, so the public now takes it for granted that the vertebrate eye has a serious “design flaw.”
“But the eye is not a watch. The human eye, though eminently functional, is imperfect ó certainly not the sort of eye an engineer would create from scratch. Its imperfection arises precisely because our eye evolved using whatever components were at hand, or produced by mutation. Since our retina evolved from an everted part of the brain, for example, the nerves and blood vessels that attach to our photoreceptor cells are on the inside rather than the outside of the eye, running over the surface of the retina.”
But in reality, the exact features critics mocked (Müller glial cells guiding light through the neural layers, the avascular foveal pit clearing the path for maximal acuity, and the one-to-one wiring of foveal cones into midget ganglion cells) are what give humans diffraction-limited vision. The so-called flaw is actually the mechanism that enables our sharpest sight. If you alter the foveal avascular zone or disrupt Müller cell waveguiding, resolution collapses, no matter how perfect the cornea or lens may be.
That’s why it’s so important to stress this point. The inverted retina isn’t just “not a flaw” — it’s a case study in how a widely repeated evolutionary talking point has turned out to be false. The real flaw is the claim that such a multilayered system arose by chance. The arrangement once used to argue against design is now recognized as essential to the system’s unmatched performance. This exposes how deeply the ‘bad design’ argument is based on shallow Darwinian assumptions rather than any serious grasp of the underlying natural technology.
The eye possesses what is called immune privilege — a special status shared with a few other organs such as the brain and testes, where immune responses are tightly regulated. In most tissues, immune attack means inflammation, but in transparent structures like the cornea and retina, inflammation would scatter light and destroy vision. To prevent this, the eye is equipped with mechanisms engineered to permit immune surveillance while avoiding destructive responses. The blood–retina barrier restricts the entry of immune cells and molecules, the aqueous humor carries suppressive factors like TGF-β, and surface cells in the cornea and retina display signals that warn the immune system not to attack. Even when foreign antigens are introduced, the reaction often produces tolerance rather than full immunity, a phenomenon known as ACAID. Immune privilege is therefore a balancing act: guarding against infection while preserving the clarity and precision that vision demands. This protective system extends further: aqueous humor must be produced and drained through the trabecular meshwork at a finely tuned rate. Small imbalances in pressure crush the optic nerve head and thin the retinal nerve fiber layer. Damage to drainage structures far from the photoreceptors still ends in field loss and blindness. The eye’s survival depends on this whole web of interdependent systems working together.
Taken together, these are irreducible aspects of one technological system. The tear film, corneal pumps, iris aperture, gradient-index lens, ciliary actuator, phototransduction cascade, retinoid regeneration, retinal signal processing, ocular motor stabilization, and reflex control loops are not a pile of interchangeable parts. Each assumes the presence of the others. Remove the tear film and the best retina sees a fog. Remove the RPE and photoreceptors starve. Remove the vestibulo-ocular reflex and the sharpest optics paint a smeared world during the smallest head movement. The eye works because the optics, biochemistry, electrophysiology, neural coding, and mechanics are co-fitted and live-serviced at millisecond to daily timescales. That is what “technologically advanced” means here: not a clever piece, but an integrated machine where the function vanishes when any essential subsystem is absent.
To say that such a system arose blindly is not only implausible but illogical. Most people, when pressed, concede that it must have been difficult, but they quickly reassure themselves that evolution “somehow” managed it. After all, we have eyes, so evolution must have done the job. What they do not realize is that the official account is not one miraculous accident, but dozens. Specialists in evolutionary biology admit that eyes as we know them today cannot be traced to a single origin. Because the designs are so radically different, the theory requires that eyes evolved independently at least forty times, and in some estimates more than sixty.
The human eye is classed as a “camera-type” eye, with a cornea and lens focusing light onto a retina. Vertebrates share this architecture. But cephalopods such as squid and octopus also have camera-type eyes, nearly identical in optical design yet inverted in structure. With no ancestral link, the claim is that the camera eye evolved twice. In jellyfish, specifically box jellies, yet another form of the camera eye appears, with lens, cornea, and retina in an animal considered primitive. Once again, the conclusion is independent invention. Insects and crustaceans have compound eyes made of thousands of repeating units, scallops have mirror eyes built on reflective crystals instead of lenses, nautilus uses a pinhole aperture, starfish spread light-sensitive cells across their arms, and chitons embed crystalline eyes directly into their shells. Each is too distinct to be explained as a modification of another. The only option left in the evolutionary framework is to multiply origins.
The public is rarely told this. Popular treatments present the story as a simple sequence: a light-sensitive patch deepened into a cup, the cup added a lens, and the lens sharpened into the eye we know today. The suggestion is that the eye evolved once, step by step. But in professional literature the concession is clear: the great differences between eye types cannot be reconciled, so the process must have happened repeatedly and independently. What ordinary people take as one improbable event is actually multiplied forty or sixty times over. Pause for a moment and let that sink in. Evolution is not claiming one miracle of accident, but forty or more times independently — when even once defies logic. It is a multiplication of implausibility.
Even when we call the eye a “camera,” the comparison flatters our devices, not the eye. The best lenses we can grind still scatter light compared to the cornea and crystalline lens, which are living, self-repairing optics that maintain transparency and alignment over decades. A camera lens must be manufactured to a fixed curvature; the human lens changes its curvature rapidly during accommodation — thickening axially and steepening the anterior surface as the ciliary body contracts, allowing focus on near objects. These changes happen in tens to hundreds of milliseconds under ideal conditions. Our sensors are rigid silicon grids; the retina is a layered neural network that not only detects photons but preprocesses the scene before a signal even leaves the eye. Edges, contrast, motion, direction, and color-opponent channels are extracted at the front end, compressing the flood of light into precisely structured streams of information. No camera sensor performs this level of computation within its pixels.
Dynamic range is another point of superiority. Human eyes can operate from starlight down to bright noon, a difference of more than a trillion-fold in light intensity, adjusting seamlessly without saturation. Artificial cameras need multiple exposures or high dynamic range algorithms to mimic even a fraction of this range. Motion stabilization is also unmatched. Tiny microsaccades refresh the retinal image, the vestibulo-ocular reflex cancels head movement in real time, and smooth pursuit locks onto moving targets. Every one of these functions requires a whole-body integration of muscles, nerves, and brain circuits that cameras cannot approximate.
And then there is repair and longevity. Digital sensors degrade quickly; their pixels burn out, lenses scratch, shutters fail. Yet eyes are serviced every moment by tear films, blink reflexes, immune privilege, molecular chaperones, and daily regeneration of photoreceptor segments. Photoreceptors themselves are among the most metabolically active cells known, consuming and renewing their own outer discs in a 24-hour cycle without ever shutting down the system.
Cameras need technicians; the eye is its own technician.
This is why biological eyes are more advanced. They are not only optical instruments, but integrated, adaptive, self-maintaining technologies. They focus, filter, stabilize, process, and transmit with a precision and resilience that our best engineering can only imitate in fragments.
What makes the eye such a devastating case against evolution is that even specialists cannot smooth over the problem. They openly concede that eyes are so different across phyla that the story of a single origin cannot be maintained. The only option left is to multiply the accident; Darwinists insist that eyes arose independently forty, fifty, or sixty times. Most people never hear this admission, but it is there in the literature. It is the equivalent of claiming that cameras, camcorders, and smartphones all evolved separately in different basements by trial and error. The “convergence” invoked as an explanation is really a poorly designed escape hatch, a way to preserve a theory rather than to face its failure.
The failures to explain the technology seen in nature are so numerous that documenting them all would fill a library. Each system reveals another point where evolutionary storytelling runs out, and the evidence points instead to design.
Natural Technology provides a more coherent reading. The reason eyes look like technology is because they are technology — living, adaptive, self-repairing optical systems. Their diversity is not a record of countless independent miracles of accident, but a showcase of different designs applied to different purposes. The human eye outstrips any camera we have built not for lack of time or refinement, but because it operates on a level of integration and foresight that neither blind trial nor our best engineering can reach.
Science will always continue to measure, to observe, and to refine. That is its strength. But when materialists crosses the line into stories of unobserved beginnings, it quietly replaces evidence with assumption. The eye exposes this sleight of hand in the most direct way. We do not see accidents multiplied until they appear designed; we see design from the start.
If you want to explore this idea in full, with examples from physics, biology, and history, I’ve written about it in detail in my books Natural Technology: The Theory of Everything and War of Cosmogonies. They take the same careful look at assumptions behind modern science and show how the evidence points consistently to design, not accident.